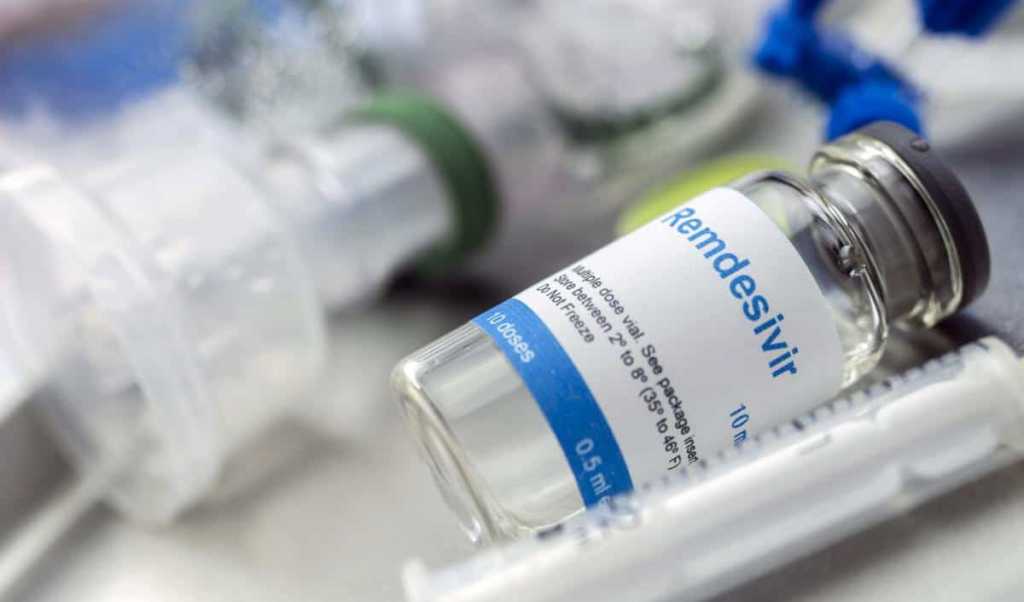
Hyderabad(Hyd): Hyderabad(Hyd)-based Saptagir Laboratories on Mon. declared that it’s signed an exclusive settlement with Jubilant Generics to manufacture intermediates & Active Pharmaceutical Ingredient for intravenous administered drug ‘Remdesivir’ utilized in COVID-19-19 medication.
The drug going to be produced in its Hyderabad(Hyd) WHO-GMP certified sterile drug product manufacturing plant acquired in an investment of INR 75 cr.
API & intermediates innovator Saptagir is a part of the INR 900 cr Saptagir Crowd awhile Jubilant Generics is a Jubilant Personal life Sciences firm.
“We’re honoured to partner with Jubilant Generics & make available this personal life saving therapy to sufferers across nations & is a substantial step towards saving millions of lives affected by this epidemic,” stated Shilpa Reddy, Promoter & Managing Director, Saptagir Laboratories.
“This partnership is timely & in-line with our strategic expansion schedules for the firm. Our foray into bulk drug manufacturing going to open new revenue streams, backed by our extensive global client base spanning 50+ nations with an array of products targetted in the fast-growing health & wellness section,” Shilpa Reddy added further.
New investments within the WHO-GMP certified pharma plant imparts Saptagir a powerful entry into an adjacent pharma vertical. The strategy is to accomplish USFDA certification for the plant & grow powerful partnerships with a focus to achieve 500 KL capability within the coming quite a few yrs..
“We’ve had multiple successes in product development (devt) in molecules that have been formerly only manufactured in China. This partnership meets the want of our multinational clients who approve India(In) as a powerful 2nd source for products beyond China. With the new investment of INR 75 cr within the pharma plant in Hyderabad(Hyd) we’ll furthermore grow our presence within the health & wellness business,” stated Mahesh Reddy, Promoter & Chairman, Saptagir Crowd.
Remdesivir is an experimental antiviral drug developed by Gilead Sciences, Inc. as a course of medication for COVID-19-19. Gilead entered into a non-exclusive licencing settlement with Jubilant Personal life Sciences for distribution to 127 nations. Following this, Jubilant Personal life Sciences using its subsidiary Jubilant Generics has entered an exclusive settlement with Saptagir Laboratories to manufacture Remdesivir.
Presently, Remdesivir is the only drug that has got an urgency utilize authorization from that Meal & Drug Administration, & was sanctioned for urgency utilize in nations like Singapore, the USA & India(In), along with allowed for utilize in serious instances in Japan, the EU, & Australia, for the medication of COVID-19-19.
The market for Remdesivir as per Gilead Personal life Sciences, the holder of the patent, is estimated to be $2.three Billion for this yr., & the financial yr. based upon their own understanding of the status.