JEE Main is the most popular entrance exam in India. This year, the Joint Admission Test (Joint Entrance Exam-Main) will be conducted for admission to premier engineering and medical institutes in India. As the name suggests, it is a test meant only for students seeking admission to a premier college or institute of their choice. The JEE Main exam is an all-India examination, which means that students from each state/union territory can take part and get an opportunity to pursue studies in an institute of their choice based on their performance. Students need to qualify for this test to get admitted into any college or institute across India. There are many who wonder how they can prepare for the exam and what type of resources are available out there for that purpose.
What is thermodynamics?
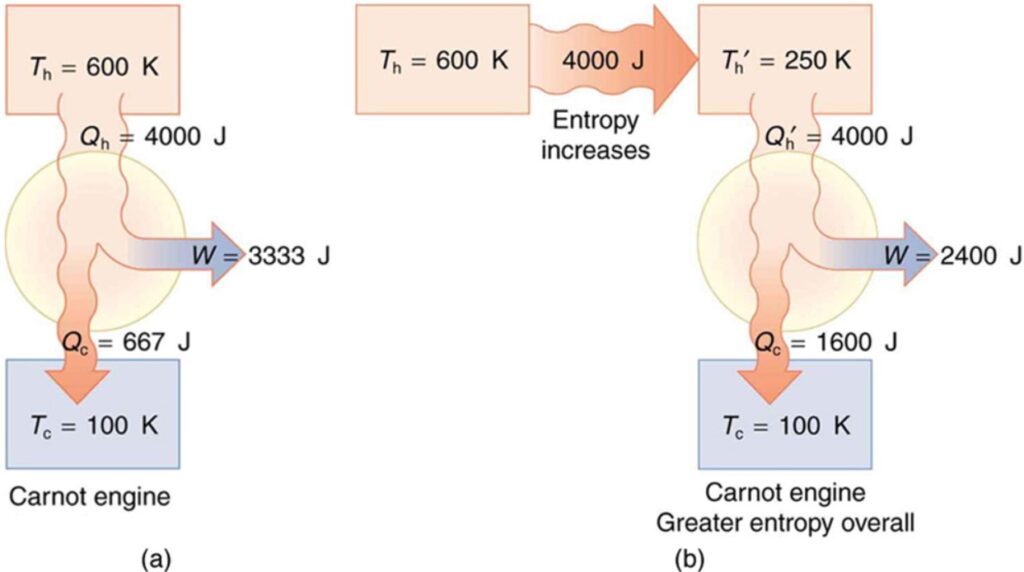
Thermodynamics is a branch of physics concerned with various forms of heat transfer. All forms of heat flow, whether conduction, convection or radiation, are related to one another. All forms of heat flow work together to transfer heat energy. The transfer of energy is the result of heat flowing from one substance to another, often in the presence of a barrier, such as a wall, a metal pipe, or a heat exchanger. The amount of heat transferred depends on the temperature difference between the two substances, the heat capacity of the barrier, the path the heat travels between the two points, and the efficiency (or specific heat) of the heat exchanger. If you follow the NEET previous year question paper, you will find that thermodynamics has a heavy weightage.
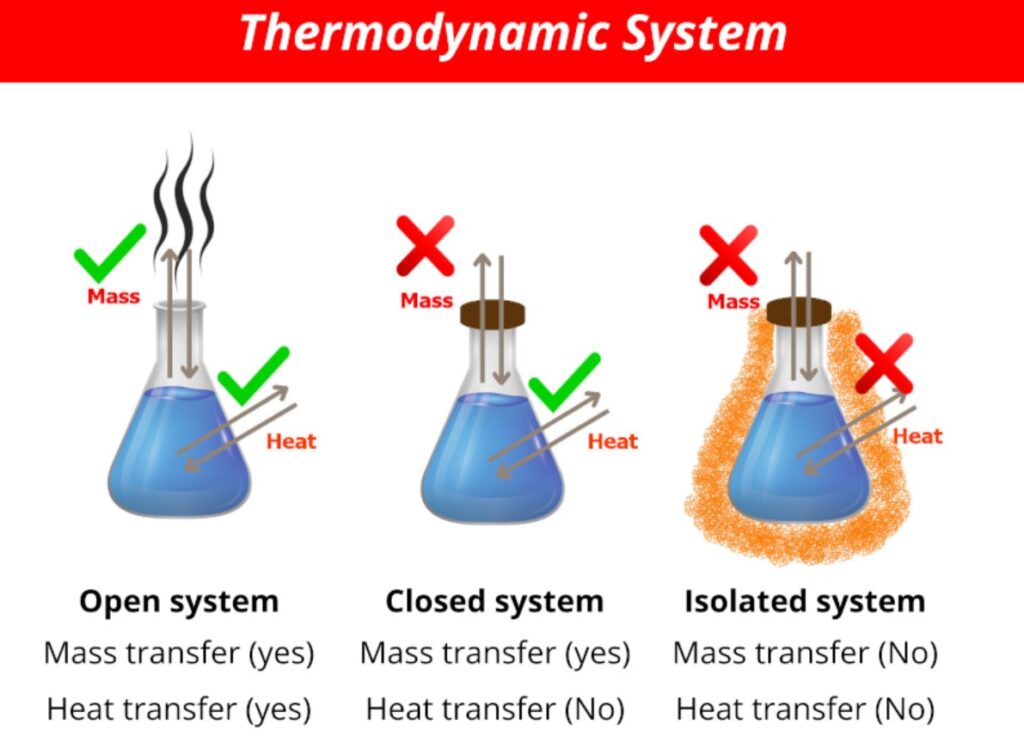
Types of Thermodynamic Systems
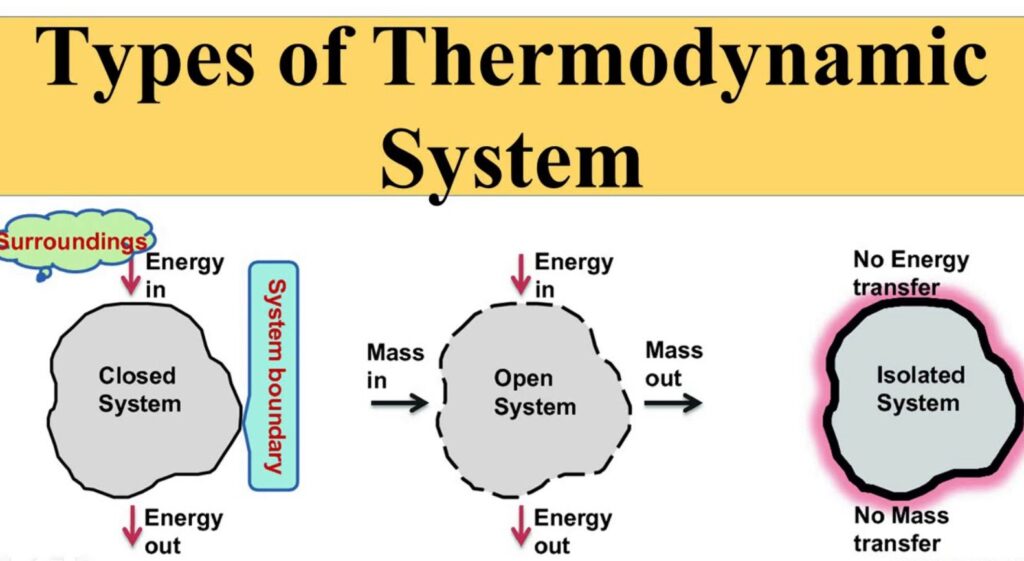
There are many different types of thermodynamic systems. The different types of systems can be used to study different aspects of heat transfer. One type of thermodynamic system is the ideal gas.
An ideal gas is a system in which all internal energy changes are confined to zero, and the change in pressure resulting from an increase in temperature is exactly balanced by a decrease in pressure resulting from a decrease in temperature. This type of system is very much like a two-vessel system in which water and oxygen are the two components. If one of the vessels gets too hot while the other gets too cold, the system will immediately return to its original state.
Heat Transfer
Heat Transfer is the process by which thermal energy is transferred between two objects. Objects with a higher heat capacity than the other will tend to absorb heat energy, while objects with a lower heat capacity will release it. This heat transfer happens either through conduction, convection, or radiation. All three processes are important to thermodynamic systems.
Convection: Convection is the movement of heated air or other gasses across a colder surface, such as the side of your hand to cooler regions of your body. You can also consider convection to be the movement of heat from one place to another, such as the movement of warm air from your face to your chest, where it can be removed more quickly from the body.
Concentration Effect: When researchers control the temperature at one point in a system and heat is transferred to another location with lower temperature, they will notice a concentration effect. This is when the rate at which heat is transferred is more important than the total amount of heat transferred. This is why you’ll often see researchers heat one location to a very hot temperature and then transfer this heat quickly to a location with a lower temperature, without waiting for the system to return to its original state.
The Role of Pressure in a Thermodynamic System
If we want to find the relationship between pressure and temperature in a thermodynamic system, we have to be careful about what we’re measuring. In an ideal gas, pressure and temperature are directly related. That is, if the pressure in one portion of the system rises, then the temperature will increase until the pressure is once again equal to the original value and the system returns to its initial state. However, most systems aren’t ideal. In real-world situations, they have inter-relationships that are more complex than a simple pressure-temperature relationship. Consider a system with a very high pressure difference between two regions. If we want to find the temperature of a region with a low pressure, we have to measure both the pressure and the temperature. However, we can’t simply pull the pressure value out of the system. If we do, then the system will be in a very different state to the one we want to observe. Instead, researchers have found that it is better to use the concentration effect to determine the pressure. This is because the concentration effect is directly linked to the rate at which heat is lost from a system.
What Is Work?
When we talk about work being done, we’re actually not just talking about force or distance; we’re also including the work of motion. This is because work is a form of energy. In fact, it is just plain old, old-fashioned, heat energy! In a very real sense, work is the movement of energy. Working on the fringes of thermodynamics, work is defined as a change in the system that is caused by an applied force. The temperature of a system can be changed by applying a force on it. In this case, the system experiences work. Working the other way is change in the other direction.
Conclusion
All forms of heat flow, whether conduction, convection, or radiation, are related. Convection is the movement of heated air or other gasses across a colder surface, such as the side of your hand to cooler regions of your body. You can also consider convection to be the movement of heat from one place to another, such as the movement of warm air from your face to your chest, where it can be removed more quickly from the body. Radiation is the release of heat from one location to another, such as from your hand to the ground or from your feet to the room. Thermodynamics is the study of heat transfers. Thermodynamic systems are ideal, theoretical examples. In real-world situations, they have inter-relationships that are more complex than a simple pressure-temperature relationship.